Abstract
Acute myeloid leukemia (AML) is an aggressive form of leukemia originating in the bone marrow (BM) with poor survival outcomes that has been largely resistant to modern cellular and targeted therapies. To improve therapeutic responses, a better understanding of how AML interacts with or evades immune cells is needed. Innate lymphoid cells (ILCs) are a recently discovered class of immune cells that exert their effector functions in an antigen-independent manner. This sentinel function of ILCs likely makes them among the first immune cell types to interact with leukemic blasts. Under homeostatic conditions, type 3 innate lymphoid cells (ILC3s) play important roles in regulating mucosal immunity through production of GM-CSF and IL-22, among others. In the setting of disease, ILC3s are not confined to mucosal tissues, as recent reports have demonstrated expansion of ILC3 populations in solid tumors (Carrega et al., 2015, Nat. Commun). However, the role of ILC3s in AML is unknown. Therefore, we sought to further study and characterize the role of ILC3s in AML biology.
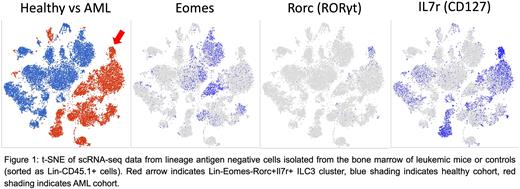
We began by utilizing a previously generated Mll-PTD;Flt3-ITD double knock-in murine model of AML that recapitulates human disease (Zorko et al., 2012, Blood). We conducted scRNA-seq analysis of the BM of leukemic mice compared to non-leukemic controls and observed clustering of a Lin-Eomes-Rorc+Il7r+ ILC3 population unique to the AML cohort that was absent in the control group (Figure 1). Furthermore, gene set enrichment analysis showed strong enrichment of an ILC3 gene signature in this cluster. We then validated this observation via flow cytometry and observed an 18.7-fold increase of ILC3s (Lin-Eomes-RORγt+CD127+) in the BM of leukemic mice relative to controls (1.31% +/- 0.20% AML vs 0.07% +/- 0.02% WT, n=5, p<0.001). Taken together, these data demonstrate ILC3 expansion in the leukemic marrow.
We next sought to determine the mechanism(s) leading to expansion of this ILC3 population in AML. We have previously implicated skewed differentiation of a common ILC precursor (ILCP) as a mechanism of NK cell dysregulation in AML (Lordo et al., 2021, J Immunol). To determine whether AML cells are capable of skewing differentiation towards ILC3s, we isolated human ILCPs from healthy donor peripheral blood and cultured them under differentiating conditions with or without the AML cell lines U937 or MV411. We observed a significant increase in ILC3 frequency when co-cultured with AML cells (59.78% +/- 3.06% with U937, 57.84% +/- 2.87% with MV411 vs 28.02% +/- 3.89% alone, n=15, p <0.001). This observation was also recapitulated in the BM of a PDX model (23.91% +/- 3.89% with MV411 vs 3.22% +/- 1.01% without MV411, n=10; p<0.0001) and further validated with single cell clonal assays. Overall, these findings suggest ILC3 expansion in AML is due at least in part to skewing of ILC development.
Finally, we wanted to characterize the functional consequences of ILC3s on AML cells. To begin to address this, we co-cultured human ILC3s (Lin-CD94-NKp44+) with the AML cell line U937 in transwells for 48 hours before subjecting the AML cells to a natural killer (NK) cell killing assay. We observed significantly reduced NK cell killing of AML cells when co-cultured with ILC3s compared to controls, suggesting ILC3s protect leukemic cells from NK-mediated killing (24.3% killing +/- 3.81% AML Alone vs 14.57% +/- 1.35% AML with ILC3, n=18, p<0.01). To determine what soluble factor(s) mediate this effect, we performed qRT-PCR on ILC3s following incubation with AML cells and found increases in GM-CSF and IL-22 expression in ILC3s relative to ILC3s cultured alone, which was also validated in our murine AML model. Furthermore, colony forming assays using the murine ILC3-like cell line MNK3 with LSKs (Lin-Sca1+Kit+) isolated from leukemic mice showed increased expression of myeloid markers (CD11b, CD11c, and Gr1) on LSK-derived cells when co-incubated with MNK3 cells relative to LSKs cultured alone. This finding suggests ILC3s may promote myeloid skewing of leukemic precursors.
Our data create a novel working model of immune evasion in AML in which ILC3 expansion occurs via AML-mediated skewing of ILC development towards ILC3s which then protect AML blasts from NK cell immune surveillance. Current studies are continuing to study the relationship between ILC3s and AML and how to target these interactions to restore AML susceptibility to immune-mediated therapeutic targeting.
Disclosures
Freud:ImmuneBridge, Inc.: Consultancy. Mundy-Bosse:Radiant Biotherapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal